Abstract
Introduction: Despite recent advances in the treatment of Hodgkin and non-Hodgkin lymphoma, hematopoietic stem cell transplantation (HSCT) remains the cornerstone of therapy for relapsed and refractory (R/R) disease. Furthermore, in Mexico, the use of targeted therapies like check point inhibitors or monoclonal antibodies is restricted by costs. In addition, information regarding outcomes after HSCT is needed but scarce. Thus, we aimed to register transplantation activity for lymphomas in Mexico. The primary objective was to assess overall survival (OS) and Progression-Free Survival (PFS). Secondary outcomes included to describe and compare the conditioning employed and differences between relapsed and refractory patients.
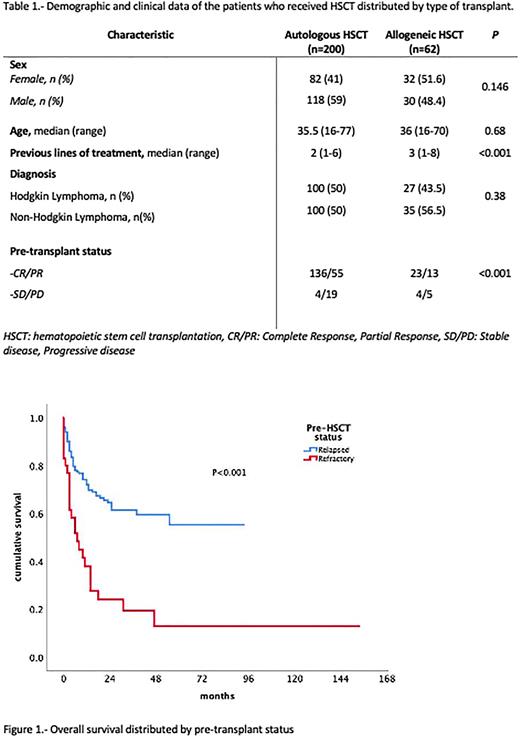
Material and methods: We conducted a retrospective analysis performed by Transplantation and Cellular Therapy Mexican working group (TCTMX), including 8 HSCT transplant centers in Mexico. Patients with HL or NHL who received HSCT from 2016 to 2021 were analyzed. Responses were assessed per the Lugano criteria. Pre-transplant status was defined as relapsed if pateints were in complete response (CR) or partial response (PR) after ≥1 line of treatment, and refractory if they had stable (SD) or progressive disease (PD). Demographic and transplantation data were collected, including the type of transplant, conditioning, maintenance, engraftment, relapse, and death. The OS and PFS were estimated using the Kaplan-Meier method.
Results: We included 262 patients from 8 HSCT centers around the country. 148 were men, and 114 were women. The median age was 36 years (range, 16-77). There was an increasing number of transplants by year (108 transplants were performed between 2016 and 2018 and 154 between 2019 and 2021). There were 135 (51.5%) NHL and 127 (48.5%) HL patients, n=227 had relapsed and n=35 refractory disease. Response before HSCT was evaluated with PET-CT in 148 cases (56.4%) and CT scan in 114 patients (43.5%). Autologous transplantation was performed in 200 (76.3%) patients, and 62 (23.7%) received allogeneic HSCT. Among allogeneic HSCT recipients, 19 (30.6%) were MSD, 42 (67.7%) were haploidentical, and 1 (1.61%) matched unrelated donor (MUD). Conditioning regimens included BEAM/BEAM-like in 90 (34.4%), melphalan-based in 87 (33.2%), busulfan-based in 36 (13.7%), and 8 (3.1%) received other regimens. Five-year OS was 79.6% for HL (92% for autologous, and 79.5% for allogeneic), and 69% for NHL (79.5% for autologous, and 43% for allogeneic). Five-year EFS was 37% for HL (72% for autologous, and 30% for allogeneic), and 50.9% for NHL (54.4% for autologous, and 38.3% for allogeneic). The main cause of death was relapse or progressive disease (n= 37, 67.2%). For refractory patients, the median OS was 30 months (95%CI: 3.3-56 months), and the median EFS was 30 months (95%CI: 3.5-6.4 months). Median OS and EFS were not reached for those in CR or PR. The OS and EFS were not significantly different between conditioning regimens.
Conclusion: Transplant activity for lymphoma in Mexico is increasing. For relapsed patients, autologous transplantation remains the best option, the lack of carmustine was not an obstacle for favorable outcomes. The prognosis for refractory patients was dismal independently of the type of transplant, and the use of new targeted therapies before transplantation is desirable in this population. Developing outcomes registries in Mexico and other Latin American countries is crucial to better guide treatment decisions in our reality.
Disclosures
Gomez-De Leon:AbbVie: Honoraria, Other: advisory board. Amador:Abbvie: Consultancy, Honoraria; BMS: Consultancy, Honoraria. García-Castillo:Roche: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Asofarma: Consultancy, Honoraria. Villela Martinez:Roche Mexico: Speakers Bureau; AstraZeneca Mexico: Speakers Bureau; Janssen Mexico: Speakers Bureau; Sanofi Mexico: Speakers Bureau; Asofarma Mexico: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.